X Chromosome Inactivation (XCI) serves as a fundamental biological process crucial for understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome. This phenomenon occurs in females, who possess two X chromosomes, only requiring the expression of genes located on one to ensure normal cellular function. Inactivation targets this excess genetic material, effectively silencing one X chromosome to prevent overexpression of X-linked genes. Recent advances in gene therapy for the X chromosome have sparked interest in therapeutic strategies that could offer new hope to those affected by these genetic conditions. With ongoing research and development of X chromosome therapies, the potential for transformative treatment options is on the horizon for many individuals living with these genetic disorders.
X Chromosome Inactivation, also known as X-inactivation, is a vital cellular mechanism that plays a key role in gene expression regulation, particularly in females. This process ensures that one of the two X chromosomes is effectively silenced, balancing gene dosage and preventing the potential overactivity associated with having two active copies. Understanding X-inactivation not only sheds light on the intricacies of cellular biology but also paves the way for innovative treatments targeting genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Novel therapeutic approaches aimed at restoring function to mutations on the X chromosome could revolutionize care and management of these conditions. As research progresses, the implications of X-inactivation extend far beyond basic science, presenting promising avenues for impactful gene therapies.
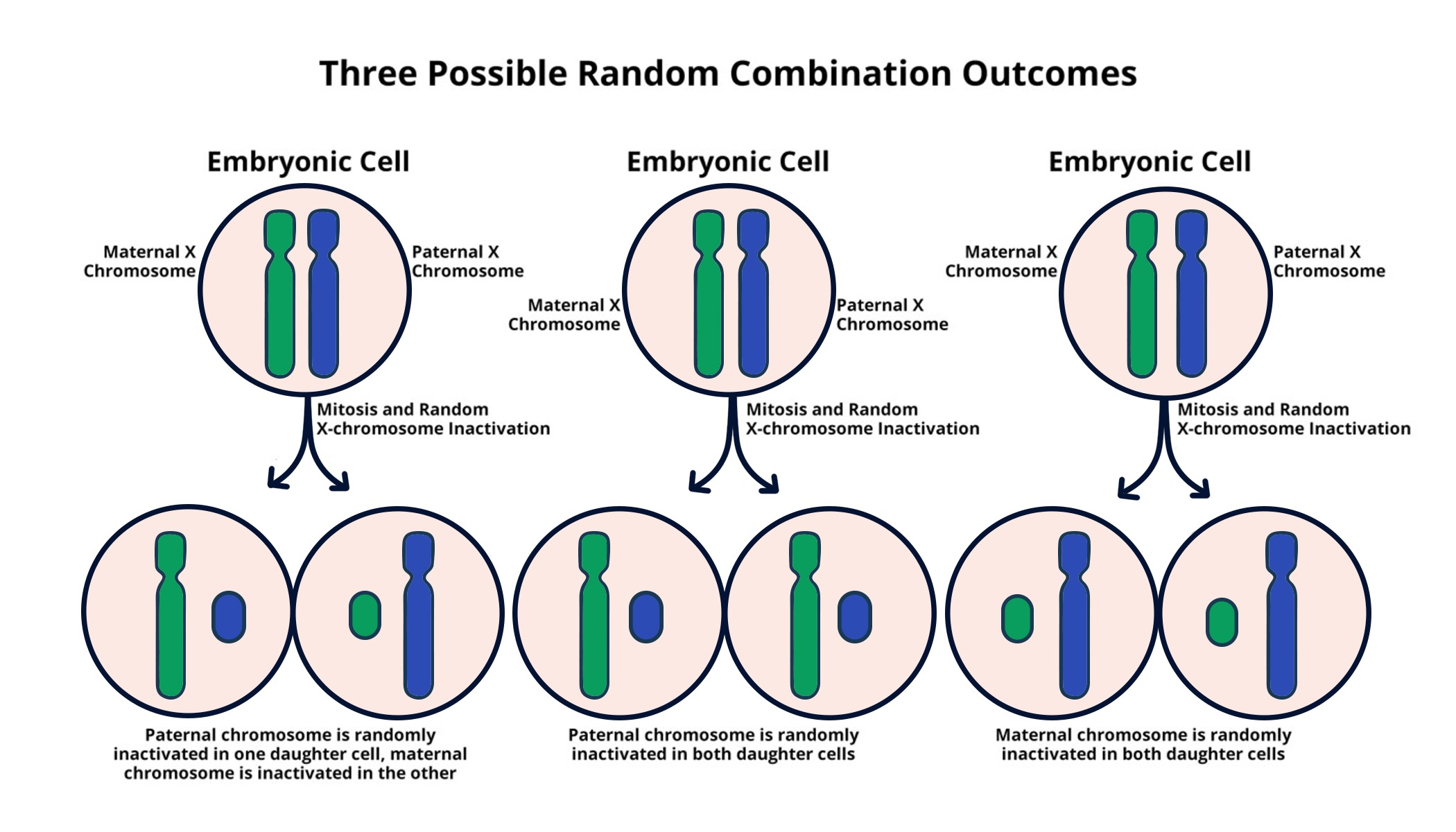
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process in females, ensuring that only one of the two copies of the X chromosome is expressed while the other is silenced. This mechanism is vital as it balances gene dosage between the sexes, preventing an overexpression of genes encoded on the X chromosome. The complex orchestration of XCI is not just a curiosity in cell biology; it has significant implications for genetic disorders linked to mutations in X-linked genes. Recent advancements, like those from Jeannie Lee’s lab, shed light on how this process works, significantly enhancing our understanding of conditions such as Fragile X Syndrome and Rett Syndrome and paving the way for targeted therapies. Understanding XCI also opens new avenues for gene therapy for X chromosome-linked diseases, potentially leading to revolutionary treatments that could alter the course of these genetic disorders.
The research conducted on X chromosome inactivation highlights how the intrinsic physical properties of chromatin, referred to as ‘Jell-O’ in Jeannie Lee’s words, play a central role in gene silencing. When the RNA molecule Xist is activated, it changes the physical state of the surrounding chromatin, creating an environment where other necessary molecules can efficiently interface with the X chromosome to initiate inactivation. These findings not only add depth to our comprehension of fundamental biological processes but also suggest innovative strategies for manipulating X chromosome behavior, thereby unlocking the therapeutic potential to treat genetic disorders like Fragile X Syndrome effectively.
Therapeutic Potential of X Chromosome Research
Research into X chromosome therapies has taken a groundbreaking turn with insights gained from studies on X chromosome inactivation. The ability to unsilence genes that have been locked away in inactive X chromosomes holds promise for treating severe genetic disorders. For instance, Fragile X Syndrome, a leading cause of inherited intellectual disability, is linked to mutations in the FMR1 gene on the X chromosome. With the knowledge gained from X-inactivation processes, researchers are exploring innovative gene therapy techniques that could reactivate the healthy gene version trapped in the inactive X chromosome, thus restoring its functionality and potentially alleviating the symptoms of the syndrome.
Similarly, Rett Syndrome, another X-linked disorder that predominantly affects females, presents a compelling case for the application of knowledge from X chromosome research. The recent focus on gene therapy for X chromosome-linked conditions emphasizes the need for safe and effective treatments. By targeting the silenced gene areas, researchers hope to develop therapies that not only release the therapeutic potential of the inactive X chromosome but also do so with minimal side effects, ensuring safety for patients. As trials progress, the dual promise of unlocking therapeutic avenues for these conditions while understanding the fundamental mechanics of X chromosome behavior could significantly impact the landscape of treating genetic disorders.
Current Advances in Gene Therapy for X Linked Disorders
Advancements in gene therapy for X-linked disorders mark a new era in the treatment of genetic diseases. The contributions from labs like Jeannie T. Lee’s are instrumental in moving from basic molecular biology to practical applications. The focus on manipulating X chromosome inactivation positions gene therapy as a key strategy, especially for conditions like Fragile X Syndrome and Rett Syndrome. With clinical trials on the horizon, the potential to not only improve lives for those suffering from these conditions but also to understand the dynamics of gene expression and regulation in human health is immense.
In the context of X chromosome-linked gene therapies, various innovative techniques are being explored. Researchers are keen on ensuring these therapies target the specific mutations responsible for disorders while preserving the integrity of the healthy genes on the same chromosome. This cautious approach is crucial, particularly since previous gene therapies have faced challenges regarding efficacy and safety. As scientists continue to decipher the complexities of X chromosome silencing and unsilencing, the prospect of effective treatments for Fragile X Syndrome and other genetic disorders could soon be within reach, marking a significant advancement in the field of genetic medicine.
Challenges and Future Directions in X Chromosome Therapies
While the advancements in understanding X chromosome inactivation are promising, challenges remain in translating this knowledge into effective therapies. One significant hurdle is ensuring the safety and effectiveness of therapies that aim to unlatch inactivated X chromosomes. There is considerable concern about the potential for unintended consequences, such as disrupting the expression of other crucial genes located on the X chromosome. This concern is particularly pertinent in the development of gene therapies as researchers strive to refine their approaches for specific targeting of mutated genes without affecting adjacent healthy ones.
Additionally, as research progresses, the logistical aspects of deploying such therapies in clinical settings also need to be addressed. Regulatory hurdles, funding for clinical trials, and the overarching need for multidisciplinary collaboration between geneticists, pharmacologists, and clinicians are vital to bringing these therapies from laboratory to bedside. Future research will not only focus on optimally applying gene therapy techniques but will also explore enhancing our understanding of the X chromosome’s roles in other potential genetic disorders. By overcoming these challenges, the therapeutic landscape for treating X-linked genetic disorders could drastically improve.
Exploring Genetic Disorders Linked to the X Chromosome
Genetic disorders linked to the X chromosome pose unique challenges and insights into human genetics. Disorders like Fragile X syndrome and Rett syndrome illustrate the complexity of gene regulation and expression on the X chromosome, particularly given its distinct pattern of inheritance. These conditions predominantly affect females, yet they also have significant implications for males who can exhibit symptoms when the corresponding mutated gene is located on their one X chromosome. Current research continues to unveil the molecular underpinnings of these conditions, particularly how identical mutations can yield varying phenotypes based on the sex of the individuals.
Understanding the genetic architecture of X-linked disorders not only aids in diagnosis and early intervention but also opens new therapeutic pathways for treatment. The exploration of gene therapy targeting specific mutations on the X chromosome is especially relevant in this context, as therapies designed to activate or replace the faulty gene could revolutionize patient outcomes. As more targeted treatments become possible, the potential for personalized medicine to address genetic disorders linked to the X chromosome is an exciting avenue that could transform the treatment landscape.
The Role of RNA Molecules in X Inactivation
RNA molecules play a pivotal role in the process of X chromosome inactivation, particularly Xist. This non-coding RNA is integral to the silencing of one X chromosome in females, and its mechanism of action has become one of the central focuses of research in genetics. Xist coats the inactive X chromosome, altering its chromatin structure and thus leading to the silencing of gene expression. Insights into how these RNA molecules interact with chromatin provide critical information that can be leveraged in developing therapies aimed at unlocking the therapeutic potential of silenced genes.
As the understanding of the role of RNA in X chromosome inactivation deepens, so does the opportunity to utilize these molecules in therapeutic contexts. Researchers are investigating the potential of employing RNA-based therapies to modulate gene expression on the X chromosome. This line of inquiry could lead to breakthroughs in treating genetic disorders such as Fragile X syndrome by allowing for the reactivation of healthy genes that are currently silenced. The future of RNA-based therapies in the context of X-linked disorders is promising, suggesting that these molecules could play a key role in the next generation of gene therapies.
Investigating Fragile X Syndrome: Current Research
Fragile X Syndrome is one of the most studied genetic disorders associated with the X chromosome, characterized by intellectual disability and developmental challenges. The condition results from a mutation in the FMR1 gene, which undergoes a unique mechanism of expansion that leads to gene silencing. Understanding how this mutation impacts the X chromosome and leads to the clinical manifestations of the disease has been at the forefront of research efforts. Innovative approaches, such as those developed by Jeannie Lee’s lab, aim to unsilence the inactive X chromosome to restore the expression of the FMR1 gene, offering a potential therapeutic avenue for those affected.
Moreover, the research into Fragile X Syndrome provides a platform for developing experimental models and testing new therapeutic strategies. By focusing on the molecular mechanisms that govern both the mutation effects and X chromosome behavior, scientists can create targeted therapies that not only address the symptoms of the syndrome but also tackle the root causes. Continued exploration into the genetic, environmental, and epigenetic factors that contribute to Fragile X will be essential in shaping future interventions and improving patient quality of life.
Rett Syndrome Research and Its Implications
Rett Syndrome is another X-linked disorder of great significance, primarily affecting females and characterized by normal early development followed by a loss of acquired skills. The genetic basis of the syndrome points to mutations in the MECP2 gene located on the X chromosome, which plays a crucial role in neural development and function. Research into the dynamics of Rett Syndrome has explored the pathways involved in gene expression regulated by MECP2, paving the way for potential gene therapy interventions that could ameliorate the disorder’s symptoms.
The implications of research into Rett Syndrome extend beyond therapeutic applications. They underscore the importance of deciphering how X-linked mutations can variably express in different individuals, even within the same family. Understanding these differences can lead to more personalized treatment approaches and better management strategies tailored to each affected individual’s needs. As ongoing research continues to elucidate the complex genetic and biochemical landscape of Rett Syndrome, the potential for unlocking new therapeutic strategies remains immensely promising.
Future Directions in Genetic Research: Gene Therapy Innovations
As advancements in genetic research continue to unfold, gene therapy innovations are at the forefront of transforming treatment approaches for X-linked disorders. The increasing understanding of regulatory mechanisms, like X chromosome inactivation, is paralleled by the development of cutting-edge techniques such as CRISPR/Cas9 that offer hope for correcting genetic mutations at their source. Researchers are now exploring how to apply these technologies to reactivate silenced genes or correct mutations, making personalized treatment possible for individuals with conditions like Fragile X Syndrome and Rett Syndrome.
Future directions in this field will likely emphasize collaborative research efforts across genetics, molecular biology, and clinical practice. Integrating patient-derived models, such as induced pluripotent stem cells (iPSCs), can provide insights into disease mechanisms and possible interventions. By bridging the gap between laboratory discoveries and clinical applications, there’s a significant potential to drive forward the development of effective gene therapy solutions for X-linked disorders, ultimately aiming to improve quality of life for individuals affected by such genetic conditions.
Frequently Asked Questions
What is X Chromosome Inactivation and why is it important in genetic disorders?
X Chromosome Inactivation (XCI) is a vital process by which one of the two X chromosomes in female mammals is silenced to ensure that gene dosage remains balanced with males, who have only one X chromosome. This mechanism is crucial in genetic disorders like Fragile X Syndrome and Rett Syndrome, as it impacts the expression of X-linked genes. By understanding XCI, researchers are exploring therapies that could potentially unmap inactive X chromosomes, which may restore function to mutated genes responsible for these disorders.
How does X Chromosome Inactivation relate to Fragile X Syndrome and Rett Syndrome?
X Chromosome Inactivation plays a significant role in diseases such as Fragile X Syndrome and Rett Syndrome because these conditions stem from mutations on the X chromosome. In females, the presence of an inactive X chromosome can trap healthy gene copies, making them unavailable for cellular function. Recent research aims to develop X chromosome therapies that could reactivate these genes, offering hope for effective treatments for individuals affected by these genetic disorders.
What advancements have been made in understanding X Chromosome Inactivation for gene therapy?
Recent studies have advanced our understanding of X Chromosome Inactivation, particularly by elucidating the cellular mechanics that enable this process. Discoveries like the role of the RNA molecule Xist in influencing the ‘Jell-O’ substance surrounding chromosomes have significant implications for gene therapy. These insights have spurred the development of innovative X chromosome therapies aimed at reactivating silenced genes, potentially offering treatments for genetic disorders including Fragile X Syndrome and Rett Syndrome.
Can X Chromosome Inactivation be targeted in X chromosome therapies?
Yes, targeting X Chromosome Inactivation is pivotal in developing X chromosome therapies. Scientists are investigating ways to manipulate this process to unsilence the inactive X chromosome, thereby allowing healthy copies of genes to express themselves in cells affected by X-linked genetic disorders. This novel approach could lead to breakthroughs in treating conditions like Fragile X Syndrome, where restoring gene function is essential.
What are the implications of X Chromosome Inactivation on gene therapy for males with X-linked conditions?
Even though males do not undergo X Chromosome Inactivation since they possess only one X chromosome, understanding this process provides insights into similar mechanisms affecting gene expression. For males with X-linked conditions like Fragile X Syndrome, targeted therapies may focus on specific mutations that cause gene silencing on the X chromosome. Research in X Chromosome Inactivation opens new paths for developing effective gene therapies that could also benefit male patients.
What future prospects are there for therapies based on X Chromosome Inactivation mechanisms?
The future prospects for therapies leveraging X Chromosome Inactivation mechanisms are promising. With ongoing research focused on unsilencing X-linked genes, researchers are optimistic about developing interventions that could treat or even cure genetic disorders such as Fragile X Syndrome and Rett Syndrome. As studies progress towards clinical trials, the potential to restore function to inactivated genes holds significant therapeutic promise for affected individuals.
| Key Points |
|---|
| The challenge of X Chromosome Inactivation in females and males due to their differing numbers of X chromosomes. |
| Jeannie T. Lee’s research has revealed the mechanism behind X-inactivation and its potential for treating X-linked disorders. |
| The gelatinous ‘Jell-O’ substance plays a vital role in influencing how the X chromosome becomes inactive. |
| Xist RNA molecule is crucial for silencing one of the two X chromosomes in females. |
| Potential treatments for Fragile X Syndrome and Rett Syndrome could free inactivated X chromosomes, restoring gene function. |
| Research indicates minimal side effects when restoring function to mutated genes without disturbing healthy genes. |
| Lee’s lab plans to move potential treatments into clinical trials by optimizing methods and conducting safety studies. |
Summary
X Chromosome Inactivation is a critical biological process, particularly in females, where one of the two X chromosomes is silenced to avoid excessive gene expression. Recent breakthroughs from Jeannie T. Lee’s laboratory not only unravel the complexities of this phenomenon but also open pathways for innovative therapies targeting genetic disorders like Fragile X and Rett syndromes. The discovery of the role of the Xist RNA molecule and its interaction with the surrounding chromosomal structure could revolutionize treatment options, offering hope for those affected by X-linked diseases. As research progresses towards clinical applications, the potential for safe and effective therapies appears promising.